- Research
- Open access
- Published:
Clinicopathologic and endoscopic characteristics of ten patients with gastric hamartomatous inverted polyp: a single center case series
BMC Gastroenterology volume 24, Article number: 139 (2024)
Abstract
Background
Gastric hamartomatous inverted polyps (GHIPs) are not well characterized and remain diagnostically challenging due to rarity. Therefore, this study aims to investigate the clinicopathologic and endoscopic characteristics of patients with GHIP.
Methods
We retrospectively reviewed clinicopathologic and endoscopic features of ten patients with GHIP who were admitted to Beijing Friendship Hospital from March 2013 to July 2022. All patients were treated successfully by endoscopic resection.
Results
GHIPs were usually asymptomatic and found incidentally during gastroscopic examination. They may be sessile or pedunculated, with diffuse or local surface redness or erosion. On endoscopic ultrasonography, the sessile submucosal tumor-type GHIP demonstrated a heterogeneous lesion with cystic areas in the third layer of the gastric wall. Histologically, GHIPs were characterized by a submucosal inverted proliferation of cystically dilated hyperplastic gastric glands accompanied by a branching proliferation of smooth muscle bundles. Inflammatory cells infiltration was observed in the stroma, whereas only one patient was complicated with glandular low-grade dysplasia. Assessment of the surrounding mucosa demonstrated that six patients (60%) had atrophic gastritis or Helicobacter pylori–associated gastritis, and four patients (40%) had non-specific gastritis. Endoscopic resection was safe and effective.
Conclusions
GHIPs often arise from the background of abnormal mucosa, such as atrophic or H.pylori-associated gastritis. We make the hypothesis that acquired inflammation might lead to the development of GHIPs. We recommend to make a full assessment of the background mucosa and H. pylori infection status for evaluation of underlying gastric mucosal abnormalities, which may be the preneoplastic condition of the stomach.
Background
Gastric hamartomatous inverted polyps (GHIPs), characterized by the downward growth of hyperplastic mucosal component into the submucosal layer [1], account for fewer than 1% of all gastric polyps [2]. They have also been called gastric inverted hyperplastic polyps(GIHPs) [3,4,5,6], because of the similarity to their colonic counterpart [7]. Collectively, lesions exhibiting inverted growth are referred to as “gastric inverted polyps (GIPs)” [2]. Kim et al. [8] divided gastric inverted polyps (GIPs) into three subtypes based on their communication with the mucosal surface, smooth muscle boundary, and tissue organization. Type 1 has a central mucosal communicating structure and a recognizable smooth muscle boundary, and has a typical round vase shape when viewed under low magnification. Half of type 1 may be accompanied by simultaneous cancer transformation. Type 2 is similar to type 1 but with no central communicating structure. Type 3 is characterized by distorted lobular tissue organization composed of cystic or hyperplastic glands and smooth muscle, without a mucosal communicating structure or smooth muscle boundary.
Because of their rarity, GHIPs are not well characterized and remain diagnostically challenging based on endoscopic findings [1]. Moreover, the pathogenesis and precancerous potential of GHIPs are still uncertain, meanwhile their association with various forms of gastritis has not been well documented in the literature. Herein we retrospectively reviewed clinical, endoscopic, and histological data of ten patients with GHIPs, all of which were resected successfully by endoscopy.
Methods
This retrospective study was approved by the Ethic Committee of Beijing Friendship Hospital, Capital Medical University (Beijing, China). Written informed consent was obtained from all patients. A total of ten patients with GHIP were included in the study from March 2013 to July 2022. None of the patients had prior gastric surgery or a family history of gastric cancer or gastrointestinal polyposis syndromes. The demographics, clinical manifestations, endoscopic and histopathological features were obtained from the patients’ medical records. Endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) was performed for GHIPs. Helicobacter pylori (H. pylori) infection status was assessed by 13C-urea breath test (UBT) (Shenzhen China National Nuclear Corporation Heidewe Biotechnology, China), past history of prior successful H. pylori eradication therapy, microscopic observations of biopsied/resected specimens, serum H. pylori antibody test, endoscopic manifestations [9], or a combination of these methods. 13C-UBT was performed in the morning after a at least of 6 h fasting, with no close (within the past 4 weeks) or concomitant medical history of proton pump inhibitors, antibiotics and bismuth, with a dosage of Urea of 75 mg, and the cut-off value to distinguish whether the 13C-UBT is positive or negative was defined as 4‰. Based on the results of these tests, we divided the patients into two groups according to the H. pylori infection status: Hp group (consisting of patients with current or past H. pylori infection) and uninfected group (consisting of H. pylori-uninfected patients).
The histopathological findings were analyzed by hematoxylin and eosin (HE) staining and immunohistochemical staining (for Mucin 6, Mucin-5AC, Mucin 2, Pepsinogen I and Desmin), including the glandular components (foveolar, fundic, cardiac/pyloric/mucous-neck, and intestinal type), the presence of epithelial dysplasia or not, and the characteristics of stroma, muscularis mucosae and background gastric mucosa. The exact sample size was a total of ten lesions from ten patients. All the submitted specimens were fixed with 10% neutral formaldehyde solution, followed by routine dehydration, paraffin embedding, tissue sectioning at a thickness of 4 µm and HE staining. An En-Vision two-step method was used for immunohistochemical labelling. The pepsinogen-I antibody was purchased from Abcam Company in the United States. Other antibodies (including Mucin 6, Mucin-5AC, Mucin 2 and Desmin) were purchased from Fuzhou Maixin Medical Technology Co., Ltd. Negative and positive controls were established for the above markers.
Subsequently, we investigated the atrophy status of the gastric mucosa surrounding/overlying each GHIP endoscopically (according to “Kimura and Takemoto's endoscopic-atrophic border scale [10, 11]”) and pathologically. In biopsy/resected specimens, mucosa with glandular atrophy or metaplasia (including focal or extensive intestinal/pseudo-pyloric metaplasia) was determined to be atrophic gastritis.
We compared the main clinical and endoscopic characteristics of GHIPs between patients with and without H. pylori infection. The statistical analysis was performed using R language Statistical Software (R 4.3.2). Fisher's precision probability test was used to compare categorical variables, and the independent-sample t test for quantitative variables.
Results
Clinical and endoscopic findings
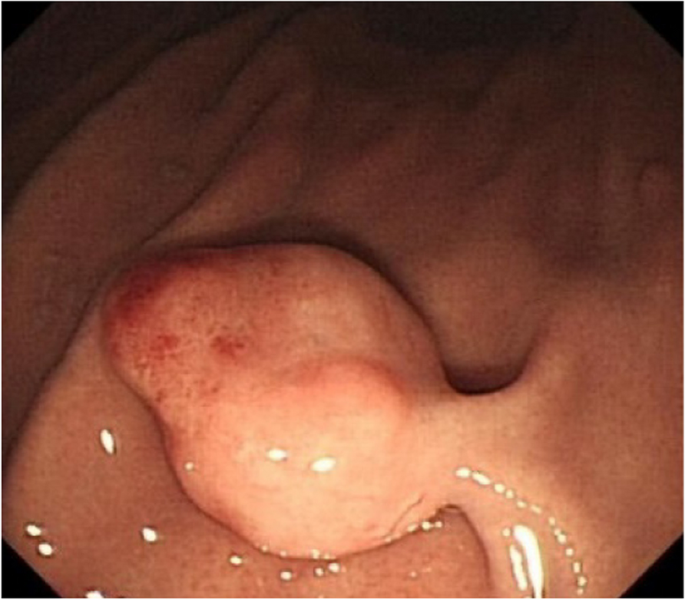
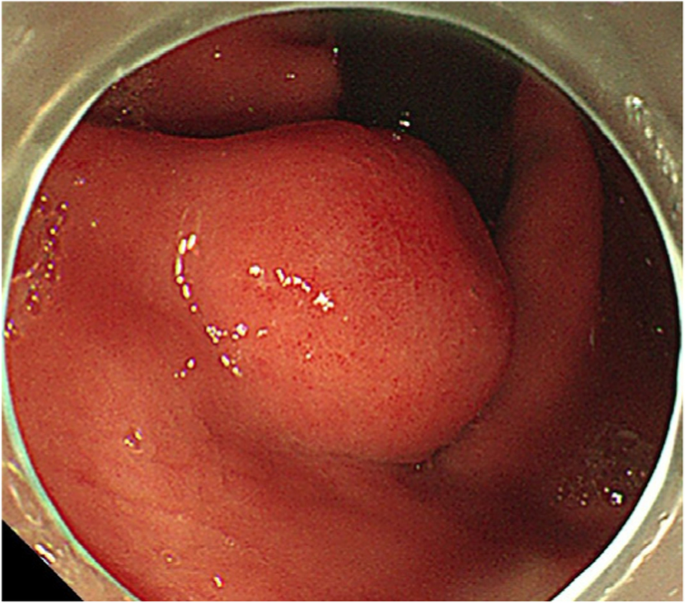
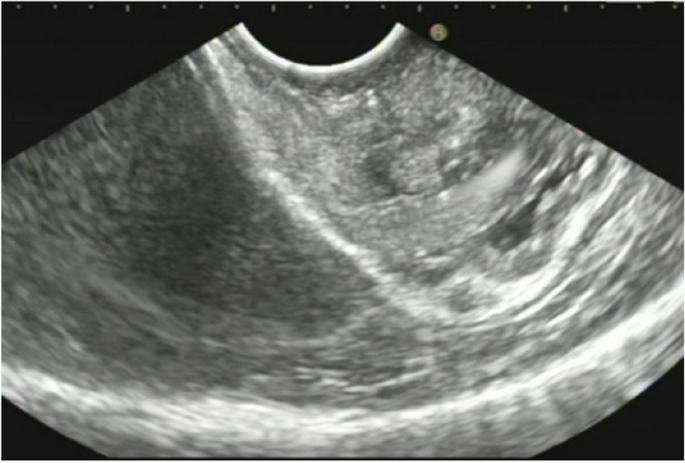
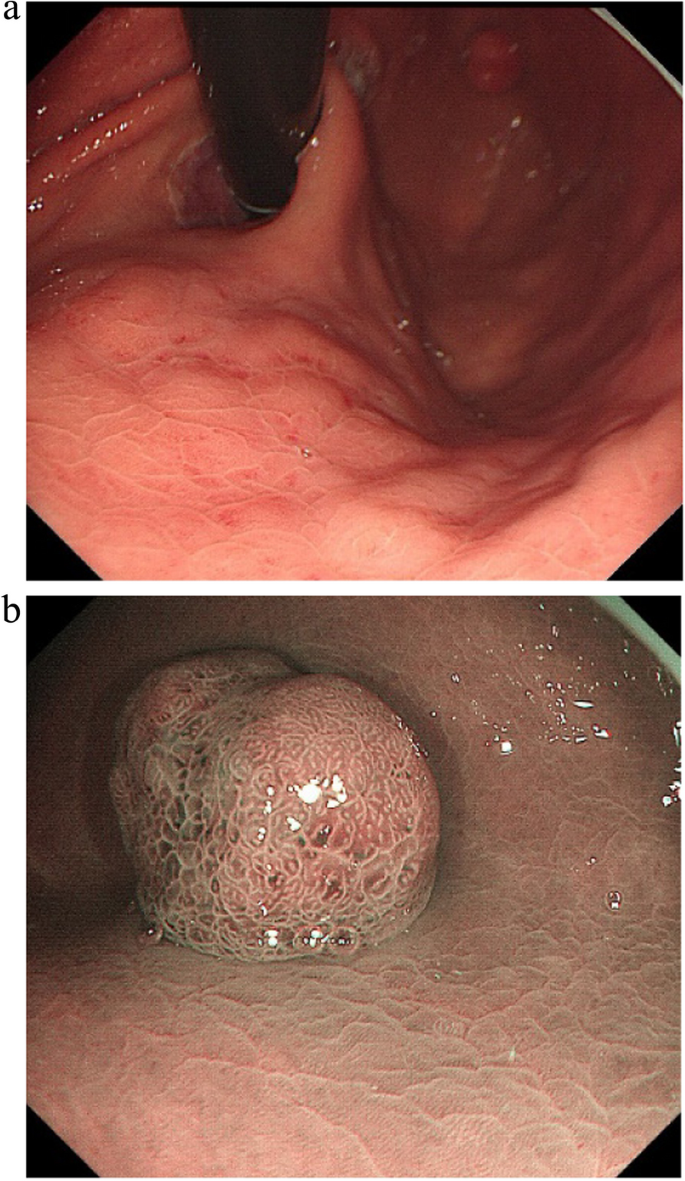
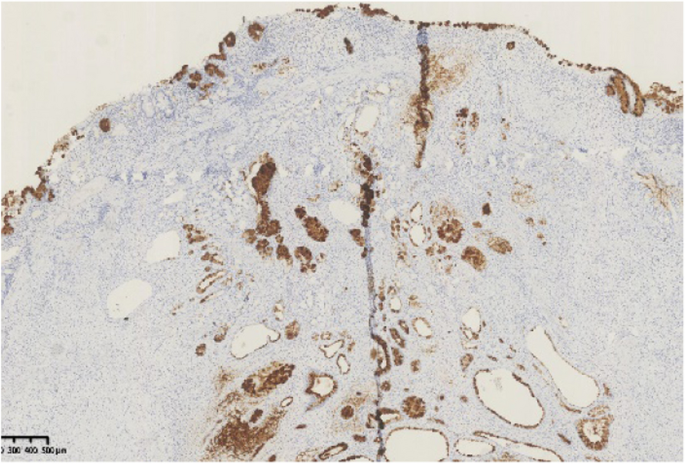
A summary of the clinical and endoscopic findings in patients with GHIP was shown in Table 1. GHIPs typically presented in late adulthood (median age of diagnosis, 59.5 years old; range, 42–79 years old), with a modest male predominance (7/10, 70%). They located in the proximal stomach (four in the middle-upper body, four in the fornix and two in the cardia), with the maximum diameter ranging from 6 to 20 mm (median size, 13.5 mm). Most patients were asymptomatic and diagnosed incidentally during endoscopic examination, however, a minority (3/10, 30%) presented with non-specific symptoms, including heartburn, acid regurgitation and epigastric distension. Endoscopically, GHIPs were solitary, and could be classified into pedunculated polyp-type (Fig. 1) (7/10, 70%), which were all completely resected by EMR, and sessile submucosal tumor (SMT)-type (Fig. 2) (3/10, 30%), which were all completely resected by ESD. All of the ten GHIPs exhibited diffuse (3/10, 30%) or local (7/10, 70%) surface redness or erosion. On endoscopic ultrasonography (EUS), all of the three sessile SMT-type GHIPs demonstrated a heterogeneous lesion with anechoic cystic areas in the third layer of the gastric wall (Fig. 3). H. pylori-infection of the gastric mucosa was confirmed in four cases (4/10, 40%), including three patients with current infection (Fig. 4a and b) and one patient with past infection. All patients were discharged without any significant complications after the endoscopic resection.
In order to investigate the correlation to Helicobacter pylori infection status, we compared the age, gender, morphology, maximum diameter and location of GHIPs between patients with and without H. pylori infection. The differences were not statistically significant (P > 0.05), as shown in Table 2.
Histopathological findings
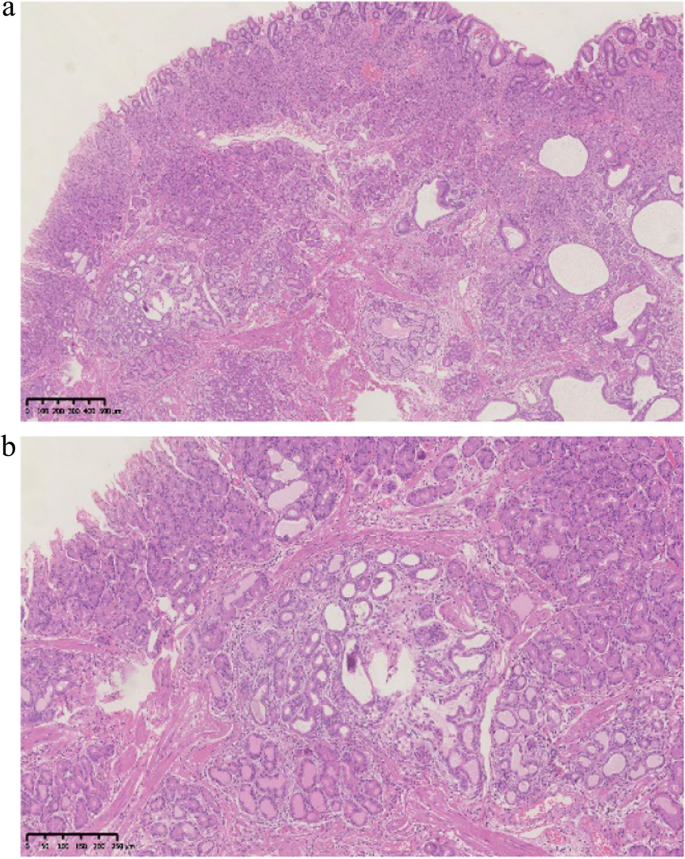
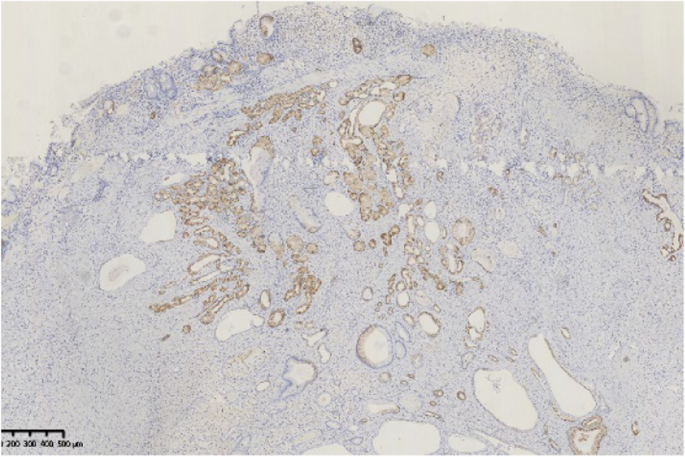
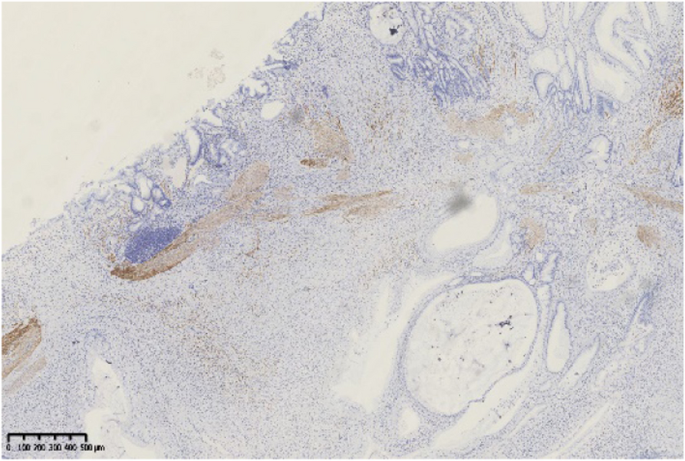
Histopathological findings in the ten GHIPs were summarized in Table 3. The histopathological examination of the ten GHIPs revealed well-circumscribed and lobulated submucosal proliferation of cystically dilated hyperplastic glands and smooth muscle bundles, partly including fibroblast cells and calcification (Fig. 5a and b). Within the GHIPs, the glandular structures mainly consisted of foveolar type (Fig. 6), cardiac/pyloric/mucous-neck type epithelium (Fig. 7), meanwhile a small quantity of fundic type or intestinal metaplasia cells were found in four cases. The continuity between the submucosal glands or cystic elements and the overlying gastric mucosa through a defect of the muscularis mucosa was observed in six GHIPs (6/10, 60%) (Fig. 8). Inflammatory cell infiltration was observed in the submucosal stroma within all the ten GHIPs. Only one GHIP (1/10, 10%) complicated with submucosal glandular low-grade dysplasia, but none was accompanied by adenocarcinoma. Assessment of the surrounding mucosa demonstrated that six patients (60%) had H. pylori–associated gastritis or atrophic gastritis with intestinal metaplasia (one of them was diagnosed as autoimmune gastritis), and four patients (40%) had non-specific gastritis.
Discussion
In the present study, we reported the clinicopathologic and endoscopic features of ten patients with GHIPs (the second largest case study of GIPs up to now). Furthermore, a literature review was conducted on previously reported cases of GIPs in PubMed using various keywords such as 'gastric inverted polyp', 'gastric inverted hyperplastic polyp', or 'gastric hamartomatous inverted polyp' between January 1978 and May 2023. To our knowledge, only 45 cases of GHIPs have been reported in English [1, 3, 5,6,7,8, 12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Table 4 summarizes the clinicopathological and endoscopic characteristics of these patients. Our review of the previously reported patients, as well as the ten present patients (totally 55 patients and 56 lesions), revealed a slight male predominance (33 males and 22 females) and a median age at diagnosis of 58 years (range: 23–81 years). Most of the patients were asymptomatic and found incidentally. However, some patients (13/55, 23.6%) presented with non-specific symptoms, including epigastric pain/discomfort, dyspepsia or heartburn and acid regurgitation. Furthermore, few patients presented with anemia secondary to chronic hemorrhage [3, 5, 14] and gastrointestinal obstruction [28] due to the lesion. The vast majority of GHIPs (54/56, 96.4%) located in the proximal stomach and the most common location was in the body (40/56, 71.4%), followed by the fundus (11/56, 19.6%), cardia (3/56, 5.4%) and antrum (2/56, 3.6%). The median diameter was 17 mm (range, 5–45 mm). The vast majority of the patients (54/55, 98.2%) had only one GHIP in the stomach, except for one patient [5] (1/55, 1.8%) who had 2 GHIPs.
The endoscopic manifestations of GHIPs were diverse. Aoki et al. [17] classified the appearances of GHIPs into sessile SMT-type and pedunculated polyp-type on endoscopy. According to this classification, sessile SMT-type was more frequently noted in GHIPs (38/56, 67.9%). Typically, the surface of GHIPs was covered with almost intact gastric mucosa, and an erosive redness or depression was frequently noted, which would indicate the relationship between GHIPs and mucosal inflammation, meanwhile a central orifice or dell with or without milky mucus outflow was occasionally observed, which would indicate the communication between submucosal lesion and gastric lumen. On EUS, the majority demonstrated a heterogeneous lesion with multiple anechoic cystic areas in the third layer of the gastric wall (23/29, 79.3%), however, a minority demonstrated a hypoechoic lesion (6/29, 20.7%). It is difficult to distinguish a GHIP with or without adenocarcinoma based on the EUS manifestations.
Furthermore, the assessment of the surrounding mucosa in the 55 patients revealed the presence of atrophic gastritis/intestinal metaplasia in 21 patients (among them 1 patient was diagnosed as autoimmune gastritis), H.pylori-associated gastritis in 10 patients, non-specific chronic gastritis in 11 patients, and data not available in 19 patients. The continuity between the submucosal glands or cystic elements and the overlying gastric mucosa through a defect of the muscularis mucosa or direct communication with the gastric mucosa was observed in 24 GIPs, suggesting that the polyp may have been formed by the heterotopic inverted downgrowth of mucosal glands into the submucosa. In addition, infiltration of chronic inflammatory cells was observed in the submucosal stroma within all GHIPs. According to all these findings, although the pathogenesis of GIPs is unknown, the heterotopic inverted downgrowth of mucosal components in GIPs is thought to develop as a result of infiltration of the mucosa through the muscularis, mucosal crevices or defects caused by repeated erosion due to various types of chronic gastritis [32]. Smooth muscle proliferated bundles would be induced by the regenerative process of both the mucosa and muscularis mucosae caused by repeated erosion [7], supporting the view of GIPs as regenerative lesions as well.
According to the classification of Kim et al. [8], the present study consisted of type 2 and type 3 GHIPs, and within the lesions no carcinomatous component was observed. However, although the exact association between gastric adenocarcinoma and GHIP is still controversial, a few studies reported GHIP coexisted with adenocarcinoma within the lesion [8, 24, 27, 33], or outside the lesion presented as synchronous or metachronous gastric adenocarcinoma [5,6,7,8, 25]. The surrounding mucosa was assessed in 9 out of the 14 patients accompanied by adenocarcinoma, revealing H. pylori–associated gastritis or atrophic gastritis with intestinal metaplasia in eight patients (8/9, 88.9%), and non-specific chronic gastritis in only one patient. All the six GHIPs coexisted with adenocarcinoma within the lesion were classified into type 1 [8], characterized by a central mucosal communicating structure, which may be the reason for neoplasia because it allowed a continuous exposure to luminal carcinogen and mechanical stress [8]. In four out of the six patients, a hyperplasia–dysplasia–carcinoma sequence was noted within the lesion [8, 24, 33], which indicated that adenocarcinoma might originate from a benign GHIP. Moreover, Ohtsu et al. [14] reported three patients with GHIP whose gene analysis demonstrated no significant mutation. It seems that GHIPs may not be premalignant lesions, but the gastric mucosa with H. pylori–associated gastritis or atrophic gastritis with intestinal metaplasia in or outside the polyp is more likely to harbor an adenocarcinoma. Therefore, one important implication of GHIPs appears to be a marker for an abnormal gastric mucosal background that is associated with the development of gastric cancer.
Moreover, as is known to all, gastrin plays a key role in gastric physiology, including various cellular processes such as proliferation, differentiation, angiogenesis, and apoptosis [34,35,36]. Gastric mucosal inflammation and hypergastrinemia, especially due to atrophic gastritis in oxyntic mucosa and H.pylori infection may play a major role in the development and neoplasia of GIPs as a result of repair, regeneration and proliferation. Additional clinicopathological studies are needed to further clarify the pathogenesis of GIPs and the association between the development of GHIPs and precancerous potential with various forms of gastritis.
In terms of treatment, endoscopic diagnosis of a GIP and neoplastic potential within a GIP may be difficult, and biopsy often faces incomplete pathological sampling of the remaining masses, therefore, complete resection may be required for subsequent pathological examination. GHIPs need to be differentiated from ectopic pancreas, gastritis cystica profunda (GCP), gastrointestinal stromal tumor (GIST) and neuroendocrine tumor (NET) [1, 17, 31], mainly through pathological characteristics. The key points of differentiation are as follows: (1) Ectopic pancreas: Microscopically pancreatic acini and ducts can help to distinguish from GHIP, (2) GCPs usually locate at the anastomotic site of the gastrointestinal tract, and the submucosal glands are composed of simple glands without obvious proliferative changes. (3) GIST and NET, as common submucosal tumors in the stomach, can be distinguished from GHIP through immune phenotypes (including immunohistochemical staining for CD34、CD117、DOG1 and Chromogranin A).The polyp-type GHIPs can be resected endoscopically by EMR, but for SMT-type especially larger than 20 mm in diameter, ESD is practical for en bloc resection currently [1, 3, 6, 13, 14, 16, 18, 19, 21, 24,25,26, 28, 29, 37], which is consistent with our present 10 cases. However, it has also been reported that laparoscopic resection may be suitable for SMT-type GHIPs with a diameter more than 20 mm [17, 23, 27, 33].
Conclusion
In summary, we present a comprehensive clinicopathologic analysis of 10 GHIPs with a literature review. GHIPs often arise from the background of abnormal mucosa, such as atrophic or H.pylori-associated gastritis. We make the hypothesis that acquired inflammation might lead to the development of GHIPs, and the gastric mucosa with H. pylori–associated gastritis or atrophic gastritis with intestinal metaplasia, whether inside or outside the polyp, is more likely to harbor an adenocarcinoma. We recommend to make a full assessment of the background mucosa and H. pylori infection status, so as to evaluate the underlying gastric mucosal abnormalities, which may be the preneoplastic condition of the stomach. Nonetheless, considering the limited number of cases and the nature of retrospective analysis in this study, further studies are needed.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
- GHIP:
-
Gastric hamartomatous inverted polyp
- GIHP:
-
Gastric inverted hyperplastic polyp
- GIP:
-
Gastric inverted polyp
- EMR:
-
Endoscopic mucosal resection
- ESD:
-
Endoscopic submucosal dissection
- H. pylori:
-
Helicobacter pylori
- UBT:
-
Urea breath test
- HE:
-
Hematoxylin and eosin
- SMT:
-
Submucosal tumor
- EUS:
-
Endoscopic ultrasonography
- LGIN:
-
Low grade intraepithelial neoplasia
- AIG:
-
Autoimmune gastritis
- AG:
-
Atrophic gastritis
- IM:
-
Intestinal metaplasia
- HAG:
-
H.pylori–associated gastritis
- NSG:
-
Non-specific gastritis
- GCP:
-
Gastritis cystica profunda
- GIST:
-
Gastrointestinal stromal tumor
- NET:
-
Neuroendocrine tumor
References
Mori H, Kobara H, Tsushimi T, Fujihara S, Nishiyama N, Matsunaga T, et al. Two rare gastric hamartomatous inverted polyp cases suggest the pathogenesis of growth. World J Gastroenterol. 2014;20:5918–23.
Sipponen P, Siurala M. Cystic, “hamartomatous” epithelial polyps of the stomach. Acta Hepatogastroenterol (Stuttg). 1978;25:380–3.
Yun JT, Lee SW, Kim DP, Choi SH, Kim SH, Park JK, et al. Gastric inverted hyperplastic polyp: A rare cause of iron deficiency anemia. World J Gastroenterol. 2016;22:4066–70.
Lee YH, Joo MK, Lee BJ, Lee JA, Kim T, Yoon JG, et al. Inverted Hyperplastic Polyp in Stomach: A Case Report and Literature Review. Korean J Gastroenterol. 2016;67:98–102.
Yamashita M, Hirokawa M, Nakasono M, Kiyoku H, Sano N, Fujii M, et al. Gastric inverted hyperplastic polyp Report of four cases and relation to gastritis cystica profunda. APMIS. 2002;110:717–23.
Lee SJ, Park JK, Seo HI, Han KH, Kim YD, Jeong WJ, et al. A case of gastric inverted hyperplastic polyp found with gastritis cystica profunda and early gastric cancer. Clin Endosc. 2013;46:568–71.
Kamata Y, Kurotaki H, Onodera T, Nishida N. An unusual heterotopia of pyloric glands of the stomach with inverted downgrowth. Acta Pathol Jpn. 1993;43:192–7.
Kim JY, Ahn S, Kin KM, Chang SH, Kim HS, Lee JH, et al. Gastric inverted polyps-distinctive Subepithelial lesions of the Stomach: Clinicopathologic analysis of 12 cases: with an emphasis on neoplastic potential. Am J Surg Pathol. 2021;45:680–9.
Kamada T, Haruma K, Inoue K, et al. Helicobacter pylori infection and endoscopic gastritis -Kyoto classification of gastritis[J]. Nihon Shokakibyo Gakkai Zasshi. 2015;112(6):982–93.
Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87–97.
Sakaki N, Arakawa T, Katou H, et al. Relationship between progression of gastric mucosal atrophy and Helicobacter pylori infection: retrospective long-term endoscopic follow-up study. J Gastroenterol. 1997;32:19–23.
Ma Q, Gao L, Sun N, Chen Y, Liu L, Liu L, et al. Gastric inverted hyperplastic polyp: A case report. Exp Ther Med. 2023;25:6.
Han YP, Min CC, Li YB, Chen YQ, Liu H, Tian ZB, et al. Diagnosis and treatment of gastric hamartomatous inverted polyp (GHIP) by endoscopic submucosal dissection: A case report. Medicine (Baltimore). 2023;102:e33443.
Ohtsu T, Takahashi Y, Tokuhara M, Tahara T, Ishida M, Miyasaka C, et al. Gastric hamartomatous inverted polyp: Report of three cases with a review of the endoscopic and clinicopathological features. DEN Open. 2023;3:e198.
Miyamoto Y, Muguruma N, Okamura S, Okada Y, Kitamura S, Okamoto K, et al. A pedunculated submucosal lesion in the stomach with inverted downgrowth. Intern Med. 2014;53:1625–8.
Hashimoto R, Okuzono T, Alahira J. An Uncommon Gastric Submucosal Tumor With Mucosal Erosion. Clin Gastroenterol Hepatol. 2018;16:e57–8.
Aoki M, Yoshida M, Saikawa Y, Otani Y, Kubota T, Kumai K, et al. Diagnosis and treatment of a gastric hamartomatous inverted polyp: report of a case. Surg Today. 2004;34:532–6.
Jung M, Min KW, Ryu YJ. Gastric inverted hyperplasic polyp composed only of pyloric glands: a rare case report and review of the literature. Int J Surg Pathol. 2015;23:313–6.
Matsui S, Kashida H, Kudo M. Gastric Inverted Hyperplastic Polyp Mimicking a Papilla. Am J Gastroenterol. 2018;113:462.
Koh J, Lee KL, Lee MS, Ahn HS, Chang MS. Gastric inverted hyperplastic polyp with inflammatory myofibroblastic tumor-like stroma, mimicking GI stromal tumor. Gastrointest Endosc. 2019;89:433–5.
Ng HI, Li ZQ, Zhang YM, Hu CF, Wang GQ. Gastric inverted hyperplastic polyp, an exceptional case diagnosed after endoscopic submucosal dissection. Clin Res Hepatol Gastroenterol. 2022;46:101890.
Noh BJ, Min JW, Sung JY, Park YK, Lee J, Kim YW. Inflammatory myofibroblastic tumor-like stromal proliferation within gastric inverted hyperplastic polyp. Pathol Int. 2016;66:180–2.
Hayase S, Sakuma M, Chida S, Saito M, Ami H, Koyama Y, et al. Diagnosis and treatment of gastric hamartomatous inverted polyp (GHIP) using a modified combination of laparoscopic and endoscopic approaches to neoplasia with a non-exposure technique (modified CLEAN-NET): a case report. Surg Case Rep. 2020;6:200.
Kim HS, Hwang EJ, Jang JY, Lee J, Kim YW. Multifocal Adenocarcinomas Arising within a Gastric Inverted Hyperplastic Polyp. Korean J Pathol. 2012;46:387–91.
Ono S, Kamoshida T, Hiroshima Y, Okawara A, Matsuo T, Kakinoki N, et al. A case of early gastric cancer accompanied by a hamartomatous inverted polyp and successfully managed with endoscopic submucosal dissection. Endoscopy. 2007;39:E202.
Dohi M, Gen Y, Yoshioka M. A case of gastric hamartomatous inverted polyp resected endoscopically. Endosc Int Open. 2016;4:E608–9.
Okamura T, Iwaya Y, Nagaya T, Muranaka F, Ota H, Umemura T. Gastric adenocarcinoma arising from hamartomatous inverted polyp during 8-year follow-up. DEN open. 2022;2:e16.
Sudo G, Tanuma T, Nakase H. Gastric Hamartomatous Inverted Polyp Causing Ball Valve Syndrome. Clin Gastroenterol Hepatol. 2019;17:e119–20.
Odashima M, Otaka M, Nanjo H, Jin M, Horikawa Y, Matsuhashi T, et al. Hamartomatous inverted polyp successfully treated by endoscopic submucosal dissection. Intern Med. 2008;47:259–62.
Carfagna G, Pilato FP, Bordi C, Barsotti P, Riva C. Solitary polypoid hamartoma of the oxyntic mucosa of the stomach. Pathol Res Pract. 1987;182:326–30.
Itoh K, Tsuchigame T, Matsukawa T, Takahashi M, Honma K, Ishimaru Y. Unusual gastric polyp showing submucosal proliferation of glands: case report and literature review. J Gastroenterol. 1998;33:720–3.
Yamagiwa H, Matsuzaki O, Ishihara A, Yoshimura H. Heterotopic gastric glands in the submucosa of the stomach. Acta Pathol Jpn. 1979;29:347–50.
Kono T, Imai Y, Ichihara T, Miyagawa K, Kanemitsu K, Ajiki T, et al. Adenocarcinoma arising in gastric inverted hyperplastic polyp: a case report and review of the literature. Pathol Res Pract. 2007;203:53–6.
Dockray GJ. Topical review Gastrin and gastric epithelial physiology. J Physiol. 1999;518:315–24.
Peach HG, Barnett NE. Determinants of basal plasma gastrin levels in the general population. J Gastroenterol Hepatol. 2000;15:1267–71.
Wroblewski LE, Pritchard DM, Carter S, Varro A. Gastrin-stimulated gastric epithelial cell invasion: the role and mechanism of increased matrix metalloproteinase 9 expression. Biochem J. 2002;365:873–9.
Yang TC, Hou MC, Chen PH, Liao WC, Li AF. Gastric hamartomatous inverted polyp mimicking ectopic pancreas on endoscopy and endosonography. Endoscopy. 2014;46:E119–20.
Acknowledgements
None.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
N.D. conceived of and performed the study, wrote the manuscript, and gave final approval of the version to be published. F.M.participated in the conception and data collection. B.Y. was responsible for the pathological analysis. J.H. conceived of and performed the study, participated in critically revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethic Committee of Beijing Friendship Hospital, Capital Medical University (Beijing, China). All the ten patients provided informed consent for treatment and participation.
Consent for publication
Not applicable. All details of participants’ images or information have been de-identified so that the identities of the patients may not be ascertained in any way.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dong, N., Meng, F., Yue, B. et al. Clinicopathologic and endoscopic characteristics of ten patients with gastric hamartomatous inverted polyp: a single center case series. BMC Gastroenterol 24, 139 (2024). https://doi.org/10.1186/s12876-024-03233-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-024-03233-8